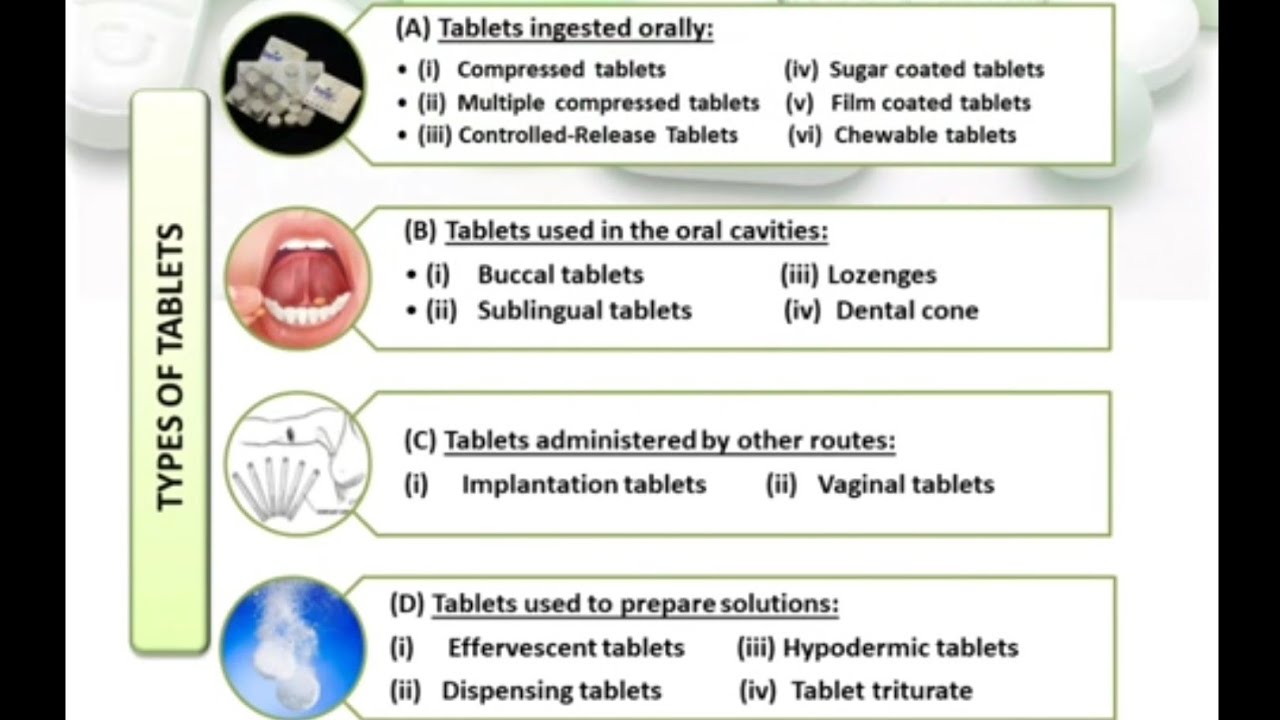
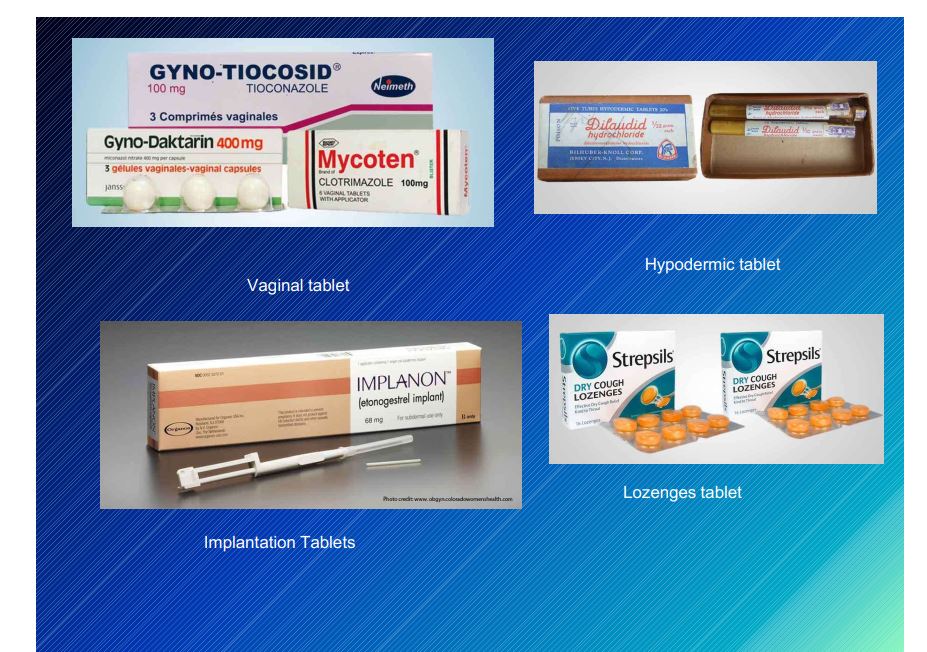
Compressed Tablets

Dies defines the shape and the size of the tablet by allowing the lower and upper punch to come close together to.
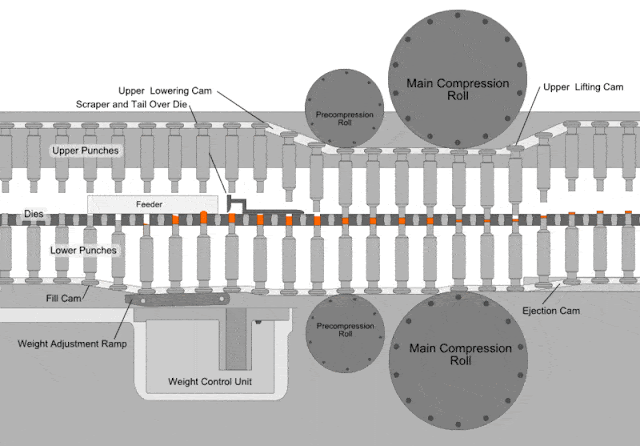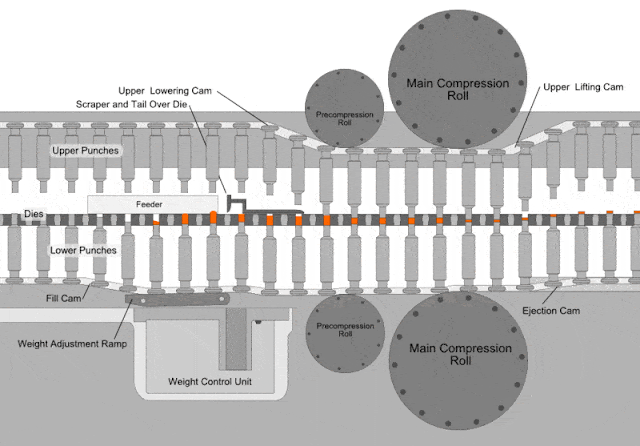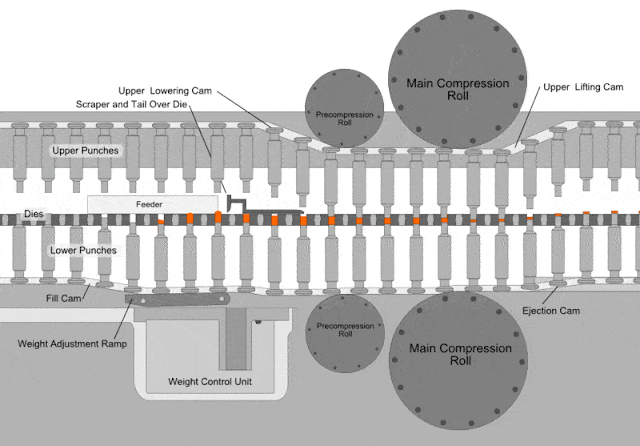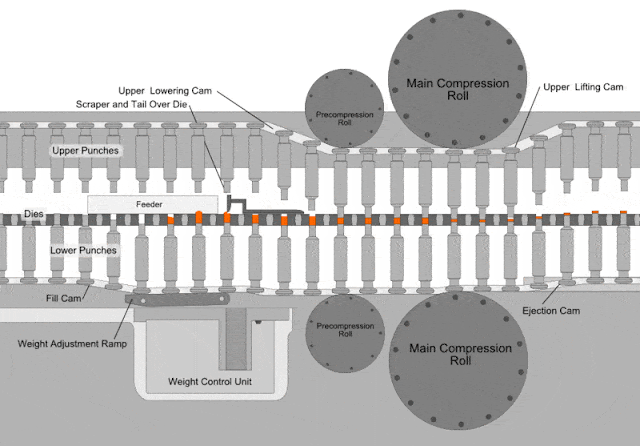
Compressed tablets. Tablet is defined as a compressed unit solid dosage form containing medicaments with or without excipients. After a quantity of powdered or granulated tableting material flow into a die the upper and lower punches of the tablet machine compress the material under a high pressure tons in2. It is usually taken orally but can be administered sublingually buccally. Parts of a single punch tablet press hopper.
The compressed tablet is the most popular dosage form in use today. According to the indian pharmacopoeia pharmaceutical tablets are solid flat or biconvex dishes unit dosage form prepared by compressing a drugs or a mixture of drugs with or without diluents. Compressed tablets are formed by compression of powdered crystalline or granular materials into the required geometry by the application of high pressures utilizing steel punches and die. About two thirds of all prescriptions are dispensed as solid dosage forms and half of these are compressed tablets.
A tablet prepared usually as a large scale production by means of great pressure. On the other hand if the materials have a good aptitude to be compressed and a good disintegrability properties the igcb will be increased with respect to the igc. It is used to hold the materials drug or the drug with excepients granules to be compressed and supply the material to the die and removes the tablet after its compression dies. The compressed tablets are made on a large scale while molded tablets are made in a short scale for experimentation or for rare use.
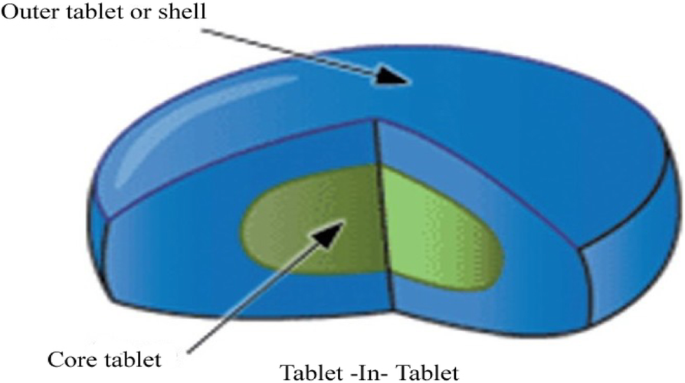
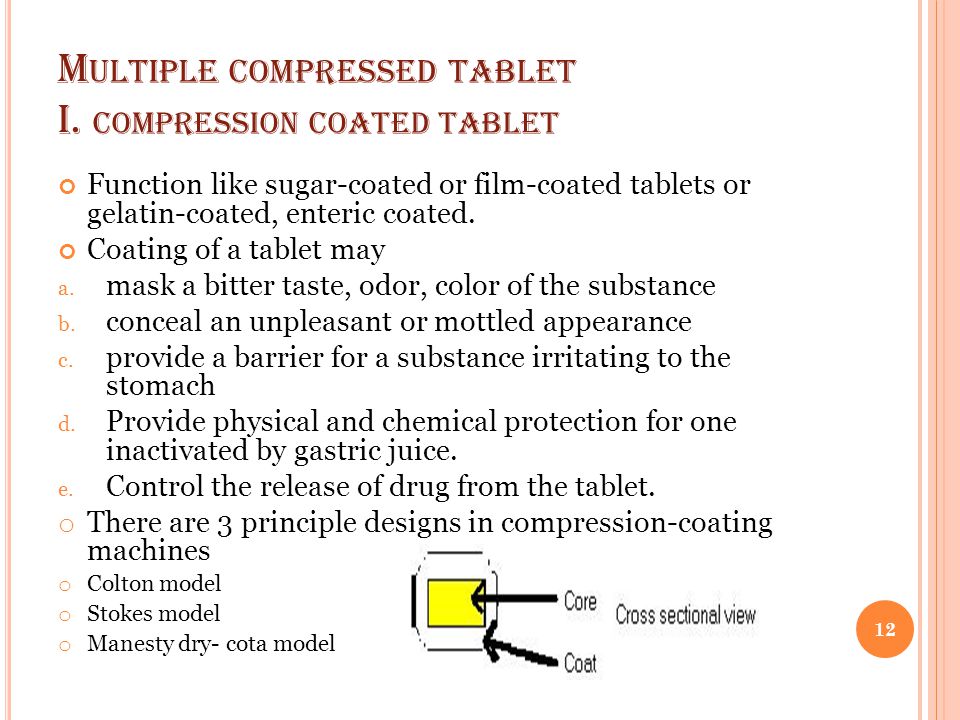
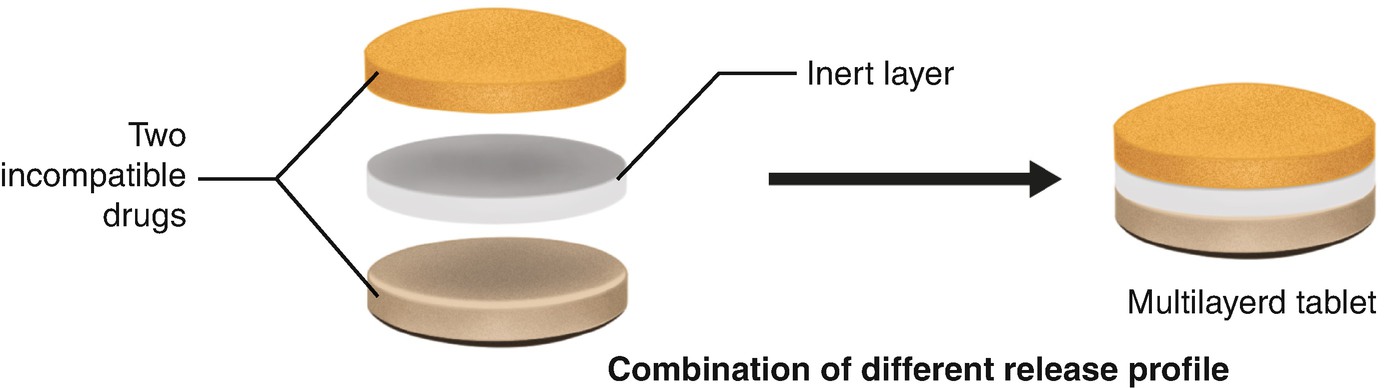
These tablets have a filmy shiny and very smooth coating on their surface. There are three subclasses of multiple compressed tablets and they include compression coated tablets layered tablets and inlay tablets. Most compressed tablets consist of the active ingredient and a diluent binder disintegrator and lubricant. Thus the sedem odt will select accurately the excipients that can be used to make compressed tablets orodispersible.
Based on surface coating of tablets. In most cases this is meant to see that the tablet is soluble in the. In addition to the active pharmaceutical ingredient s apis compressed tablets usually contain a number of pharmaceutical excipients e g bulking. The tablets were stored in an open dish at 25 c 65 rh for 6 h.
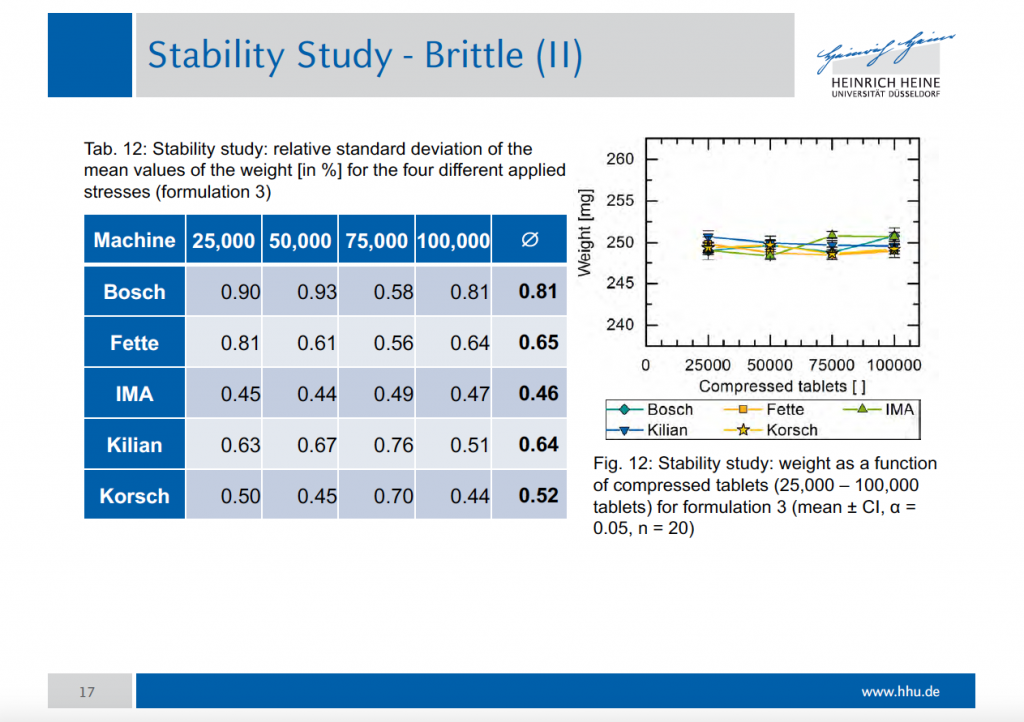
Multiple compressed tablets can also be used when there is a need to mask the bitter taste of a drug substance or where the drug substance in question is irritant to the stomach. Tablet hardness decay was evaluated using 10 tablets from each of the experimental runs on the korsch 6 station press compressed to the target hardness of 6 scu.